biotechrabbit™ Long Range DNA Polymerase is a first-choice for amplification of targets up to 40 kb in size with higher accuracy than Taq DNA polymerase.
This specially designed blend of thermophilic polymerases is well suited for amplification of targets that are GC-rich and have complex structures.
For the most demanding applications, the supplied 5× PCR Enhancer can be optionally used to improve results when using templates with GC-rich sequences and complex structures.
Long Range DNA Polymerase produces a mixture of A-tailed and blunt-end PCR products. It is advisable to blunt products before cloning into blunt-end vectors.
Component | Composition |
Long Range PCR Enzyme Mix | Long Range PCR Enzyme Mix, 2.5 U/µl, in storage buffer containing 50% (v/v) glycerol |
10× Long Range Reaction Buffer | Optimized PCR reaction buffer including magnesium ions |
5× PCR Enhancer | Proprietary PCR enhancer mix |
STORAGE | −20°C (until expiry date – see product label) |
Unit Definition
One unit catalyzes the incorporation of 10 nmol of deoxyribonucleotides into a polynucleotide fraction in 30 minutes at 72°C.
Quality Control
Functional assay
Human genomic DNA was amplified using the DNA Polymerase and specific primers to produce a distinct band of 750 bp.
Self-priming activity
Standard PCR is carried out without primers, using the DNA Polymerase and human genomic DNA. No products were amplified.
Exonuclease assay
Linearized lambda/HindII fragments are incubated with the DNA Polymerase in a 50 µl reaction mixture for 4 h at 37°C. No degradation of DNA was observed.
Endonuclease assay
lambda DNA is incubated with the DNA Polymerase in a 50 µl reaction mixture for 4 h at 37°C. No degradation of DNA was observed.
Nick Activity
Supercoiled plasmid DNA is incubated with the DNA Polymerase in a 50 µl reaction mixture for 4 h at 37°C. No conversion of covalently closed circular DNA to nicked DNA was detected.
E. coli DNA contamination assay
A sample of the denatured DNA Polymerase is analyzed with specific primers targeting the 16S rRNA gene in qPCR for the presence of contaminating E. coli DNA. No E. coli DNA was detectable.
Prevention of PCR contamination
When assembling the amplification reactions, care should be taken to eliminate the possibility of contamination with undesired DNA.
- Use separate clean areas for preparation of samples and reaction mixtures and for cycling.
- Wear fresh gloves. Use sterile tubes and pipette tips with aerosol filters for PCR setup.
- Use only water and reagents that are free of DNA and nucleases.
- With every PCR setup, perform a contamination control reaction that does not include template DNA.
Standard PCR setup
The standard PCR protocol using biotechrabbit reaction buffer provides excellent results for most applications. Optimization might be necessary for certain conditions, such as the amplification of long targets, high GC or AT content, strong template secondary structures or insufficient template purity. In such cases, optimization of template purification (see biotechrabbit nucleic acid purification kits), primer design and annealing temperature is recommended.
The best conditions for each primer-template can be optimized with the following:
- Choosing the optimal quantities of template and primers
- Determining optimal concentrations of the enzyme and magnesium ions
- Optimizing cycling conditions
If unspecific amplification occurs, the amount of DNA Polymerase and the primer concentration can be reduced. Correspondingly, these can be increased when yield is low.
Basic Protocol
- Optionally, use the supplied 5× PCR Enhancer to increase the yield and to lower the background in more complicated PCR reactions (low amounts of template, impure or GC-rich template).
- Thaw on ice and mix all reagents well, especially the MgCl2 solution and dNTPs.
- Keep all reagents and reactions on ice.
- When setting up multiple reactions, prepare a master mix of water, buffer, dNTPs and polymerase. Prepare enough master mix for one more than the actual number reactions. Alternatively, use biotechrabbit Long Range PCR Master Mix, 2× (cat. no. BR0300401)
- Pipet the master mix into thin-walled 0.2 ml PCR tubes.
- Add template and primers separately if they are not used in all reactions.
|
Component |
Volume |
Final concentration |
|
10× Long Range Reaction Buffer |
5 µl |
1× |
|
5× PCR Enhancer (optional - BR1900201) |
10 µl |
1× |
|
10 mM dNTP Mix |
1 µl |
200 µM |
|
Forward primer |
Variable |
0.2–1 µM |
|
Reverse primer |
Variable |
0.2–µM |
|
Template DNA |
Variable |
10 pg–1 μg |
|
|
Use 0.01–1 ng for plasmid or phage DNA and 0.1–1 μg for genomic DNA |
|
|
Long Range PCR Enzyme Mix (2.5 U/µl) |
0.5–1 µl |
1.25–2.5 U |
|
Nuclease free water |
Variable |
|
|
Total volume |
50 µl |
|
- Mix and centrifuge briefly to collect the liquid in the bottom of the tube.
- Place in the PCR cycler.
Cycling Program
|
Step |
Temperature |
Time |
Cycles |
|
Initial activation |
95°C |
2 min |
1 |
|
Denaturation |
95°C |
30 s |
25–35 |
|
Annealing |
55°C |
30–45 s |
25–35 |
|
|
Approximately 5°C below Tm of primers |
||
|
Extension |
72°C |
30 s/kb |
25–35 |
|
For fragments longer than 5 kb, use 68°C extension temperature and 1 min/kb timing. |
|||
|
Final extension |
72°C |
5 min |
1 |
|
|
To extend all incomplete PCR products |
||
|
Storage in the cycler |
4°C |
Indefinitely |
1 |
- Add loading dye solution (see DNA Loading Dye, 6×, cat. no. BR0800301) to the reactions to analyze PCR products on a gel or store them at −20°C.
- For cloning, always purify the PCR product from a gel (see BR0700401 GenUP™ Gel Extraction Kit).
You may also be interested in the following product(s)

RTS pIVEX Wheat Germ His6-tag Vector Set
Package Sizes |
RTS Amino Acid Sampler
Package Sizes |
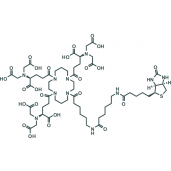
Tris-NTA Biotin
Package Sizes |
GenUP™ FFPE Paraffin Removal Solution
Package Sizes |
RTS 500 Wheat Germ CECF Kit
Package Sizes |
