biotechrabbit™ RNase Inhibitor is an acidic protein that is a potent inhibitor of a wide spectrum of ribonucleases. The RNase Inhibitor helps to prevent RNA degradation in applications like cDNA synthesis, RT-PCR, in vitro transcription/ translation reactions or RNA purification. The enzyme is purified from a recombinant E. coli strain carrying the RNase Inhibitor gene.
Component | Composition |
RNase Inhibitor | RNase Inhibitor, 40 U/µl, in storage buffer containing 50% (v/v) glycerol. |
STORAGE | -20°C (until expiry date – see product label) |
Unit Definition
One unit is defined as the amount of enzyme required to inhibit 50% of the activity of 5 ng RNase A (hydrolysis of cyclic cytidine-monophosphoric).
Quality Control
Protein Purity
The Protein purity is analyzed by SDS polyacrylamide gel electrophoresis.
RNase Assay
A sample of the enzyme was incubated with a RNA template. RNase activity was not observed after agarose gel electrophoresis.
General guidelines
- RNase Inhibitor can be used in all common reactions performed with RNA, it is active in all common buffers.
- The recommended final concentration for RNA protection is about 1 -2 units of RNase Inhibitor for every 1 µl of the reaction mixture.
- Optimal temperature for Ribonuclease Inhibitor activity is 37°C
- Inactivation conditions: 65°C for 20 minutes
Prevention of cDNA synthesis reaction contamination
RNase contamination is an exceptional concern when working with RNA. RNase A, providing most threat to RNA integrity, is a highly stable contaminant of any laboratory. To prevent RNA from degradation and to minimize possibility of contamination during cDNA synthesis; follow the guidelines below:
- Use separate clean areas for preparation of the samples and the reaction mixture.
- DEPC-treat all tubes and pipette tips or use certified nuclease-free labware with aerosol filters.
- Wear fresh gloves when handling RNA and all reagents.
- Always assess the integrity of RNA prior to cDNA synthesis in denaturing agarose gel electrophoresis.
- Use RNase free water and other reagents.
- To prevent RNA from degradation, add Ribonuclease inhibitor (optional) in to the cDNA synthesis reaction (20 units for 20 µl reaction).
Typical cDNA synthesis reaction set up
- Thaw on ice and mix very well all reagents.
- Assemble and keep all reactions on ice.
- To use time and reagents effectively, always prepare master mix for multiple reactions. For a master mix volume, always calculate the number reactions that you need plus one additional.
- Combine the following in an RNase-free reaction tube:
Component | Volume | Final concentration |
dNTP Mix (10 mM each dNTP) | 2 µl | 1 mM (each dNTP) |
RNase Inhibitor, 40 U/µl | 0.5 µl | 1 U/µl |
Oligo (dT)12–18 (10 µM) – or Hexamer Primer (25 µM) – or Gene Specific Primer (10 µM) | 0.5 µl 1 µl 0.5 µl | 0.25 µM 1.25 µM 0.25 µM |
RNA Template | 0.1–1 µg total RNA or 50–500 ng mRNA (polyA) |
|
Reverse Transcriptase, 200 U/µl | 1 µl | 10 U/µl |
5× RT Buffer | 4 µl | 1× |
RNase-free water | Variable |
|
Total volume | 20 µl |
|
- Mix and collect the drops by centrifuging briefly.
- When using
- Hexamer Primer, incubate 10 minutes at 30°C followed by 50–55°C for 20–60 minutes
- Oligo (dT) or gene-specific Primer incubate at 50–55°C for 20–60 minutes.
- Inactivate enzymes at 99°C for 5 minutes.
- Collect the drops by spinning briefly.
- Store products at –20°C or proceed to next step, like PCR or qPCR.
- Use maximum 10 µl of the cDNA synthesis reaction mix for PCR in 50 µl volume.
You may also be interested in the following product(s)
dNTP Sets
Package Sizes |
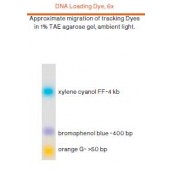
6X DNA Loading Dye
Package Sizes |
RTS 100 E. coli HY Kit
Package Sizes |
RTS pIX4.0 Insect Vector
Package Sizes |

RTS pIVEX Wheat Germ His6-tag Vector Set
Package Sizes |
